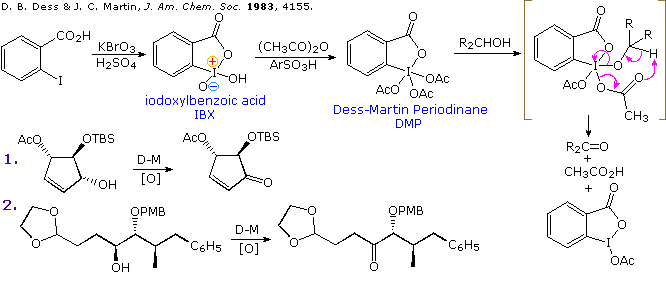
Full article: Unusual synthesis of azines and their oxidative degradation to carboxylic acid using iodobenzene diacetate
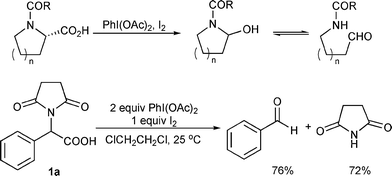
A one-pot oxidative decarboxylation–Friedel-Crafts reaction of acyclic α-amino acid derivatives activated by the combination of iodobenzene diacetate ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B815227F

Novel 2,2,6,6‐Tetramethylpiperidine 1‐Oxyl–Iodobenzene Hybrid Catalyst for Oxidation of Primary Alcohols to Carboxylic Acids - Yakura - 2011 - Advanced Synthesis & Catalysis - Wiley Online Library

Synthesis of α-sulfonyloxyketones via iodobenzene diacetate (PIDA)-mediated oxysulfonyloxylation of alkynes with sulfonic acids - RSC Advances (RSC Publishing) DOI:10.1039/C7RA11875A

PDF) ChemInform Abstract: Regiospecific Oxidation of Polycyclic Aromatic Phenols to Quinones by Hypervalent Iodine Reagents
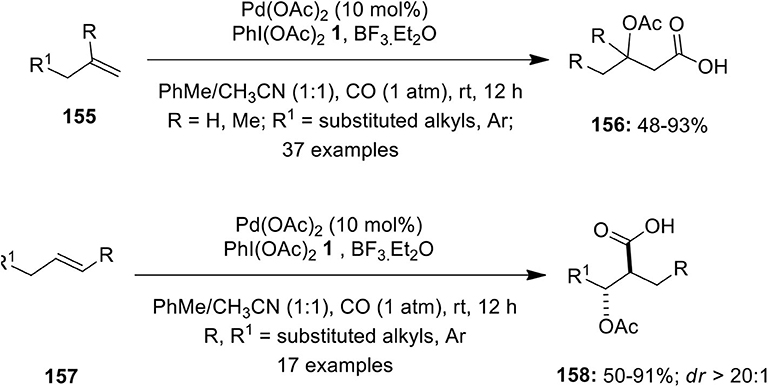
Frontiers | Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions | Chemistry

Recent Advances in Iodosobenzene‐Mediated Construction of Heterocyclic Scaffolds: Transition‐Metal‐Free Approaches and Scope - Gayen - 2018 - European Journal of Organic Chemistry - Wiley Online Library

organic chemistry - Mechanism of oxidative dearomatisation with hypervalent iodine - Chemistry Stack Exchange
![A facile iodine(III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations | Organic and ... A facile iodine(III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations | Organic and ...](https://media.springernature.com/lw685/springer-static/image/art%3A10.1186%2F2191-2858-1-1/MediaObjects/13588_2011_Article_1_Figa_HTML.gif)
A facile iodine(III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations | Organic and ...
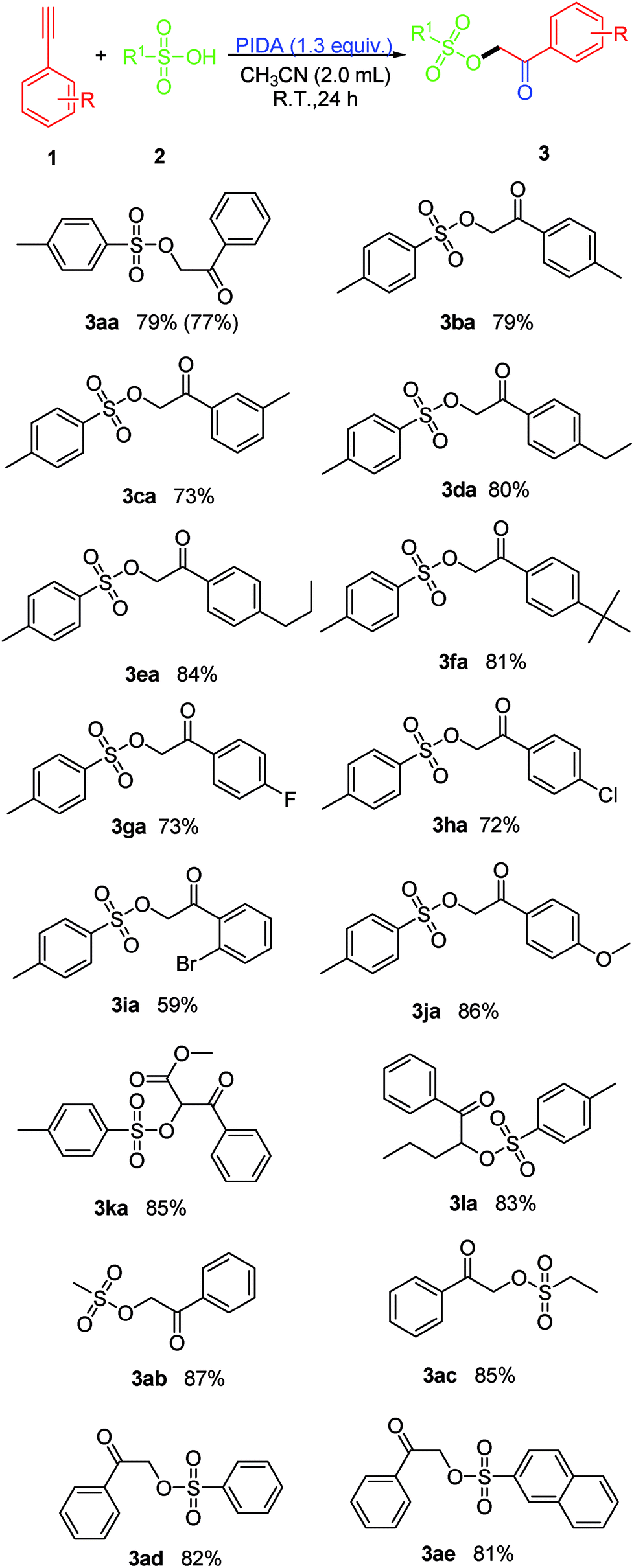
Synthesis of α-sulfonyloxyketones via iodobenzene diacetate (PIDA)-mediated oxysulfonyloxylation of alkynes with sulfonic acids - RSC Advances (RSC Publishing) DOI:10.1039/C7RA11875A

Iodine(III) promotes cross-dehydrogenative coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds | Communications Chemistry

Iodine(III) promotes cross-dehydrogenative coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds | Communications Chemistry







/8F1232C6EE59D103802585F90071D7DB/$file/FI10879_structure.png)