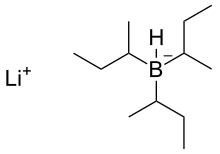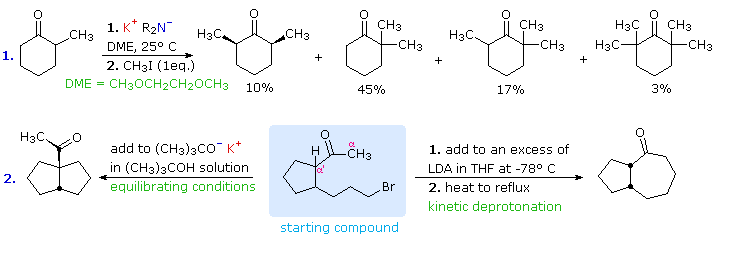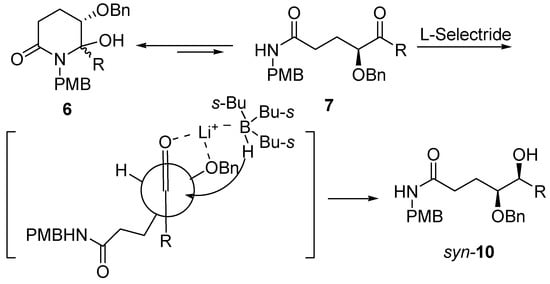
Molecules | Free Full-Text | A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | HTML

L-selectride-mediated highly diastereoselective asymmetric reductive aldol reaction: access to an important subunit for bioactive molecules. - Abstract - Europe PMC
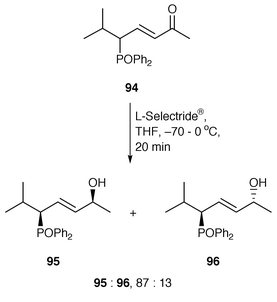
The control of remote asymmetric centres via reduction of acyclic carbonyl functions - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B000155O

Aspects of stereocontrol in the L-Selectride reduction of 4-acyl-1,3-dioxolane derivatives - ScienceDirect
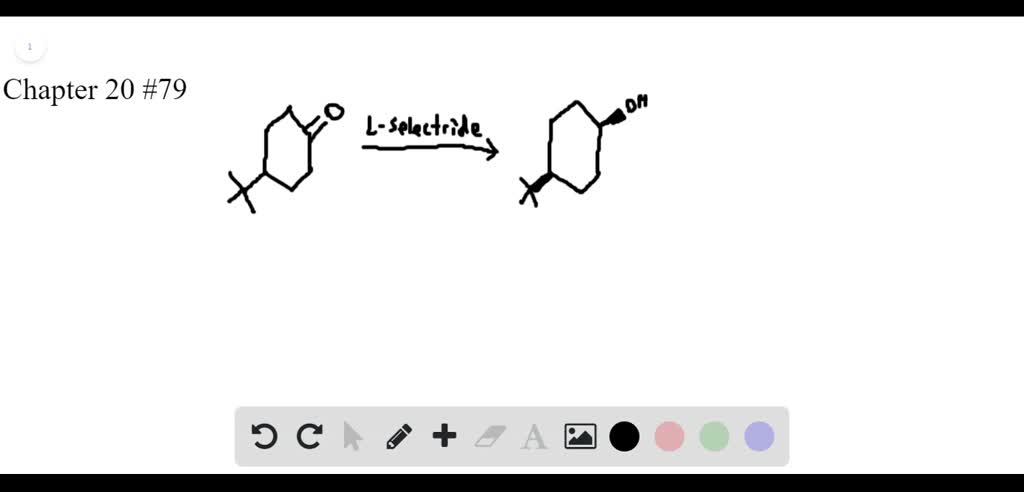
SOLVED:Lithium tri-sec-butylborohydride, also known as L-selectride, is a metal hydride reagent that contains three sec-butyl groups bonded to boron. When this reagent is used to reduce cyclic ketones, one stereoisomer often predominates

PDF) A General and Simple Diastereoselective Reduction by L-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides

L-selectride-mediated highly diastereoselective asymmetric reductive aldol reaction: access to an important subunit for bioactive molecules. - Abstract - Europe PMC

Stereoselective syntheses of galanthamine and its stereoisomers by complementary Luche and L-selectride reductions - ScienceDirect

Aspects of stereocontrol in the L-Selectride reduction of 4-acyl-1,3-dioxolane derivatives - ScienceDirect
![PDF] A General and Simple Diastereoselective Reduction by l-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | Semantic Scholar PDF] A General and Simple Diastereoselective Reduction by l-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/087e7f6d7a3505c3d28e2af3fe8dac1a991c50c9/4-Figure2-1.png)
PDF] A General and Simple Diastereoselective Reduction by l-Selectride: Efficient Synthesis of Protected (4S,5S)-Dihydroxy Amides | Semantic Scholar
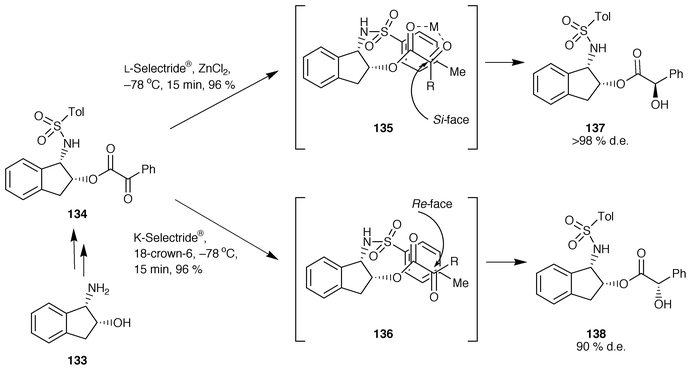
The control of remote asymmetric centres via reduction of acyclic carbonyl functions - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B000155O

MCQ about K-selectride reduction: For exams like, CSIR-NET, GATE, IIT-JAM, BARC, BS-MS, B.Sc, M.Sc. - YouTube